Summary
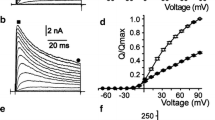
Ca2+-activated K+ channels from rat brain synaptosomal membranes were incorporated into planar lipid bilayers, and the effects of aminoglycoside antibiotics on the single channel conductance (258±13 pS at 100mm K+) were investigated. Aminoglycosides reduced the single channel conductance from the ‘cis’ (cytoplasmic) side in a dose- and voltage-dependent manner. Voltage dependence of the blockade indicated an interaction between positively charged amino residues of aminoglycoside antibiotics and a binding site located within the electric field of the ion-conducting pathway. The order of blocking potency was consistent with that of the number of amino residues of aminoglycosides (neomycin (6)>dibekacin (5)>ribostamycin (4)=kanamycin (4)), while the electrical distance (zδ=0.46–0.49) of the binding site kept almost constant for each drug. Thesezδs were almost the same with those (0.46–0.51) of alkyldiamine blockers with two amino residues (total net charge of +2) and approximately twice of those (0.25–0.26) of alkylmonoamine blockers (total net charge of +1). Assuming that amino residues of aminoglycosides and alkylamines shared the same binding site located at 25% voltage drop from the cytoplasmic surface of the channel, the site would have to be at least large enough to accommodate one diamino sugar residue of the aminoglycoside in order to simultaneously interact with two positively charged amino groups. Dose- and voltage-dependent blockade of the channel by gallamine, an extremely bulky trivalent organic cation, supported the picture that the channel has a wide mouth on the cytoplasmic side and its ‘pore’ region, where voltage drop occurs, may also be quite wide and nonselective, suddenly tapering to a constriction where most charged cations block the channel by ‘occluding’ the K+-conducting pathway.
Similar content being viewed by others
References
Armstrong, C.M. 1971. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons.J. Gen. Physiol. 58:413–437
Bartschat, D.K., Blaustein, M.P. 1985a. Potassium channels in isolated presynaptic nerve terminals from brain.J. Physiol. (London) 361:419–440
Bartschat, D.K., Blaustein, M.P. 1985b. Calcium-activated potassium channels in isolated presynaptic nerve terminals from rat brain.J. Physiol. (London) 361:441–457
Cecchi, X., Wolff, D., Alvarez, O., Latorre, R. 1987. Mechanisms of Cs+ blockade in a Ca2+-activated K+ channel from smooth muscle.Biophys. J. 52:707–716
Coronado, R., Miller, C. 1982. Conduction and block by organic cations in a K+-selective channel from sarcoplasmic reticulum incorporated into planar phospholipid bilayers.J. Gen. Physiol. 79:529–547
Coronado, R., Rosenberg, R.L., Miller, C. 1980. Ionic selectivity, saturation, and block in a K+-selective channel from sarcoplasmic reticulum.J. Gen. Physiol. 76:425–446
Eisenman, G., Latorre, R., Miller, C. 1986. Multi-ion conduction and selectivity in the high-conductance Ca2+-activated K+ channel from skeletal muscle.Biophys. J. 50:1025–1034
Farley, J., Rudy, B. 1988. Multiple types of voltage-dependent Ca2+-activated K+ channels of large conductance in rat brain synaptosomal membranes.Biophys. J. 53:919–934
French, R.J., Shoukimas, J.J. 1981. Blockage of squid axon potassium conductance by internal tetra-n-alkylammonium ions of various sizes.Biophys. J. 34:271–291
Golowasch, J., Kirkwood, A., Miller, C. 1986. Allosteric effects of Mg2+ on the gating of Ca2+-activated K+ channels from mammalian skeletal muscle.J. Exp. Biol. 124:5–13
Gray, M.A., Tomlins, B., Montgomery, R.A.P., Williams, A.J., 1988. Structural aspects of the sarcoplasmic reticulum K+ channel revealed by gallamine block.Biophys. J. 54:233–239
Gustin, M., Hennessey, T.M. 1988. Neomycin inhibits the calcium current of Paramecium.Biochim. Biophys. Acta 940:99–104
Hudspeth, A.J., Kroese, A.B.A. 1983. Voltage-dependent interaction of dihydrostreptomycin with the transduction channels in bullfrog sensory hair cells.J. Physiol. (London) 345:66P
Jones, D.H., Matus, A.I. 1974. Isolation of synaptic plasma membrane from brain by combined flotation-sedimentation density gradient centrifugation.Biochim. Biophys. Acta 356:276–287
Jordan, P.C. 1984a. Effect of pore structure on energy barriers and applied voltage profiles: I. Symmetrical channels.Biophys. J. 45:1091–1100
Jordan, P.C. 1984b. Effect of pore structure on energy barriers and applied voltage profiles: II. Unsymmetrical channels.Biophys. J. 45:1101–1107
Jordan, P.C. 1986. Ion channel electrostatics and the shapes of channel proteins.In: Ion Channel Reconstitution. C. Miller, editor. pp. 37–55. Plenum, New York
Kroese, A.B.A., Das, A., Hudspeth, A.J. 1989. Blockage of the transduction channels of hair cells in the bullfrog's sacculus by aminoglycoside antibiotics.Hear. Res. 37:203–218
Krueger, B.K., French, R.J., Blaustein, M.B., Worley, J.F. 1982. Incorporation of Ca2+-activated K+-channels from rat brain into planar lipid bilayers.Biophys. J. 37:170a
Krueger, B.K., Worley, J.F., French, R.J. 1983. Single sodium channels from rat brain incorporated into planar lipid bilayer membranes.Nature (London) 303:172–175
Latorre, R. 1986. The large calcium-activated potassium channel.In: Ion Channel Reconstitution. C. Miller, editor. pp. 431–467. Plenum, New York
Latorre, R. 1980. Varieties of calcium-activated potassium channels.Annu. Rev. Physiol. 51:385–399
Miller, C. 1982. Bis-quarternary ammonium blockers as structural probes of the sarcoplasmic reticulum K+ channel.J. Gen. Physiol. 79:861–891
Moczydlowski, E., Alvarez, O., Vergara, C., Latorre, R. 1985. Effect of phospholipid surface charge on the conductance and gating of a Ca2+-activated K+ channel in planar lipid bilayers.J. Membrane Biol. 83:273–282
Moczydlowski, E., Latorre, R. 1983. Gating kinetics of Ca2+-activated K+ channels from rat muscle incorporated into planar bilayers.J. Gen. Physiol. 82:511–542
Nelson, M.T., French, R.J., Krueger, B.K. 1983. Voltage-dependent calcium channels from rat brain incorporated into planar lipid bilayers.Nature (London) 308:77–80
Nelson, M.T., Roudna, M., Bamberg, E. 1983. Single K+-channel current measurements from brain syaptosomes in lipid bilayers.Am. J. Physiol. 245:C151-C156
Neyton, J., Miller, C. 1988a. Potassium blocks barium permeation through a calcium-activated potassium channel.J. Gen. Physiol. 92:549–567
Neyton, J., Miller, C. 1988b. Descrete Ba2+ block as a probe of ion occupancy and pore structure in the high-conductance Ca2+-activated K+ channel.J. Gen. Physiol. 92:569–586
Nomura, K., Naruse, K., Sokabe, M. 1989. Aminoglycoside blockade of Ca-activated K channel from rat brain synaptosomal membrane.Proc. Int. Union Physiol. Sci. 17:259
Oberhauser, A., Alvarez, O., Latorre, R. 1988. Activation by divalent cations of a Ca2+-activated K+ channel from skeletal muscle membrane.J. Gen. Physiol. 92:67–86
Ohmori, H. 1985. Mechano-transduction currents in isolated vestibular hair cells of the chick.J. Physiol. (London) 359:189–217
Oosawa, Y., Sokabe, M. 1986. Voltage-dependent aminoglycoside blockade of the sarcoplasmic reticulum K+ channel.Am. J. Physiol. 250:C361-C364
Reinhart P.H., Chung, S., Levitan, B. 1989. A family of calcium-dependent potassium channels from rat brain.Neuron 2:1031–1041
Rudy, B. 1988. Diversity and ubiquity of K channels.Neuroscience 25:729–749
Sokabe, M. 1983. Neomycin blockade of K+ channels from sarcoplasmic reticulum membranes incorporated in planar bilayers.Proc. Jpn. Acad. 59B:33–37
Sokabe, M. 1984. Aminoglycoside blockade of cation channels from polyphosphoinositides and sarcoplasmic reticulum in planar bilayer membranes.In: Transmembrane Signaling and Sensation. F. Oosawa, H. Hayashi, and T. Yoshioka, editors. pp. 119–134. Japan Science Society Press/VNU Science Press BV, Utrecht
Sokabe, M., Hayase, J., Miyamoto, K. 1982. Neomycin-effect on lysotriphosphoinositide channel as a model for an acute ototoxicity.Proc. Jpn. Acad. 58B:177–180
Squire, L.G., Petersen, O.H. 1987. Modulation of Ca2+-and voltage-activated K+ channels by internal Mg2+ in salivary acinar cells.Biochim. Biophys. Acta 899:171–175
Suarez-Kurtz, G., Reuben, J.P. 1987. Effects of neomycin on calcium channel currents in clonal GH3 pituitary cells.Pfuegers Arch. 410:517–523
Swenson, R.P. 1981. Inactivation of potassium current in squid axon by a variety of quarternary ammonium ions.J. Gen. Physiol. 77:255–271
Tachibana, M., Komiya, S., Yamada, C., Sokabe, M. 1984. Correlation of ototoxic effect of polyaminocompounds in vivo with effect on triphosphoinositide metabolism and lysotriphosphoinositide channel in vitro.Proc. Jpn. Acad. 60B:318–321
Tanifuji, M., Sokabe, M., Kasai, M. 1987. An anion channel of sarcoplasmic reticulum incorporated into planar lipid bilayers: Single-channel behavior and conductance properties.J. Membrane Biol. 99:103–111
Vergara, C., Moczydlowski, E., Latorre, R. 1984. Conduction, blockade and gating in a Ca2+-activated K+-channel incorporated into planar lipid bilayers.Biophys. J. 45:73–76
Villarroel, A., Alvarez, O., Oberhauser, A., Latorre, R. 1988. Probing a Ca2+-activated K− channel with quartanary ammonium ions.Pfluegers Arch. 413:118–126
Wagner, J.A., Snowman, A.M., Olivera, B.M., Snyder, S.H. 1987. Aminoglycoside effects on voltage-sensitive calcium channels and neurotoxicity.N. Engl. J. Med. 317:1669
Wong, B.S., Adler, M. 1986. Tetraethylammonium blockade of calcium-activated potassium channels in clonal anterior pituitary cells.Pfluegers Arch. 407:279–284
Woodhull, A.M. 1973. Ionic blockage of sodium channels in nerve.J. Gen. Physiol. 61:687–708
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nomura, K., Naruse, K., Watanabe, K. et al. Aminoglycoside blockade of Ca2+-activated K+ channel from rat brain synaptosomal membranes incorporated into planar bilayers. J. Membrain Biol. 115, 241–251 (1990). https://doi.org/10.1007/BF01868639
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01868639