Summary
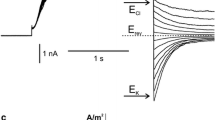
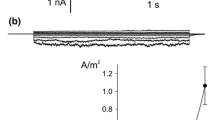
The mechanisms of Cl−-channel activation in the plasmalemma ofNitellopsis obtusa was studied by measuring both the transient inward current under voltage clamp and Cl− efflux during the action potential. 9-anthracenecarboxylic acid (A-9-C) at 1.0mm inhibited both the transient inward current and the Cl− efflux, but did not uncouple the sudden cessation of the cytoplasmic streaming. Since this excitation-cessation coupling is caused by a transient increase in the cytoplasmic Ca2+ concentration, these results suggest that A-9-C inhibited not the Ca2+ channel but specifically the Cl− channel. The following results were found between the Ca2+-channel activation and the Cl−-channel activation: (1) The Ca2+-channel blocker La3+ uncoupled the excitation-cessation coupling and inhibited both the transient inward current and the Cl− efflux, although the Cl−-channel blocker A-9-C did not affect the excitation-cessation coupling. (2) The Cl− efflux was greatly reduced by depletion of Ca2+ from the external solution and restored by an increase in the external Ca2+ concentration. (3) An increase in the external ionic, strength which increases Ca2+ entry (T. Shiina & M. Tazawa,J. Membrane Biol. 96:263–276, 1987) enhanced the Cl− efflux. (4) Mg2+, which cannot pass through the Ca2+ channel, reduced both the transient inward current and the Cl− efflux. (5) Although Sr2+ can pass through the plasmalemma Ca2+ channel, Cl−-channel activation by Sr2+ was only partial. These findings support the hypothesis that voltage-dependent Ca2+-channel activation, which increases the free Ca2+ concentration in the cytoplasm, is necessary for the subsequent Cl−-channel activation.
Similar content being viewed by others
References
Bader, C.R., Bertrand, D., Schwartz, E.A. 1982. Voltage-activated and calcium-activated currents studied in solitary rod inner segments from the salamander retina.J. Physiol. (London) 331:253–284
Barish, M.E. 1983. A transient calcium-dependent chloride current in the immatureXenopus oocyte.J. Physiol. (London) 342:309–325
Barry, W.H. 1968. Coupling of excitation and cessation of cyclosis inNitella: Role of divalent cations.J. Cell. Physiol. 72:153–160
Beilby, M.J. 1984. Calcium and plant action potentials.Plant Cell Environ. 7:415–421
Beilby, M.J., Coster, H.G.L. 1979. The action potential inChara corallina. II. Two activation-inactivation transients in voltage clamps of the plasmalemma.Aust. J. Plant Physiol. 6:323–335
Bryant, S.H., Morales-Aguilera, A. 1971. Chloride conductance in normal and myotonic muscle fibers and the action of monocarboxylic aromatic acids.J. Physiol. (London) 219:367–383
Caldwell, J.H., Brunt, J. van, Harold, F.M. 1986. Calcium-dependent anion channel in the water mold,Blastocladiella emersonii.J. Membrane Biol. 89:85–97
Coleman, H.A. 1986. Chloride currents inChara—A patch-clamp study.J. Membrane Biol. 93:55–61
Evans, M.G., Marty, A. 1986. Calcium-dependent chloride currents in isolated cells from rat lacrimal glands.J. Physiol. (London) 378:437–460
Findlay, G.P. 1961. Voltage-clamp experiments withNitella.Nature (London) 191:812–814
Findlay, G.P. 1970. Membrane electrical behaviour inNitellopsis obtusa.Aust. J. Biol. Sci. 23:1033–1045
Findlay, G.P., Hope, A.B. 1964. Ionic relations of cells ofChara australis. IX. Analysis of transient membrane currents.Aust J. Biol. Sci. 17:400–411
Findlay, I., Petersen, O.H. 1985. Acetylcholine stimulates a Ca2+-dependent Cl− conductance in mouse lacrimal acinar cells.Pfluegers Arch 403:328–330
Gaffey, C.T., Mullins, L.J. 1958. Ion fluxes during the action potential inChara.J. Physiol. (London) 144:505–524
Hayama, T., Shimmen, T., Tazawa, M. 1979. Participation of Ca2+ in cessation of cytoplasmic streaming induced by membrane excitation in Characeae internodal cells.Protoplasma 99:305–321
Hope, A.B. 1961. The action potential in cells ofChara.Nature (London) 191:811–812
Hope, A.B., Findlay, G.P. 1964. The action potential inChara.Plant Cell Physiol. 5:377–379
Kikuyama, M. 1986. Tonoplast action potential of Characeae.Plant Cell Physiol. 27:1461–1468
Kikuyama, M. 1987. Ion efflux during a single action potential ofNitella axilliformis in medium lacking Ca2+.Plant Cell Physiol. 28:179–186
Kikuyama, M., Oda, K., Shimmen, T., Hayama, T., Tazawa, M. 1984 Potassium and chloride effluxes during excitation of Characeae cells.Plant Cell Physiol. 25:965–974
Kikuyama, M., Tazawa, M. 1983. Transient increase of intracellular Ca2+ during excitation of tonoplast-freeChara cells.Protoplasma 117:62–67
Kishimoto, U. 1964. Current voltage relations inNitella.J. Physiol. (London) 14:515–527
Lunevsky, V.Z., Zherelova, O.M., Vostrikov, I.Y., Berestovsky, G.N. 1983. Excitation ofCharaceae cell membranes as a result of activation of calcium and chloride channels.J. Membrane Biol. 72:43–58
Mailman, D.S., Mullins, L.J. 1966. The electrical measurement of chloride fluxes inNitella.Aust. J. Biol. Sci. 19:385–398
Marty, A., Tan, Y.P., Trautmann A. 1984. Three types of calcium-dependent channel in rat lacrimal glands.J. Physiol. (London) 357:293–325
Mayer, M.L. 1985. A calcium-activated chloride current generates the after-depolarization of rat sensory neurones in culture.J. Physiol. (London) 364:217–239
Miledi, R. 1982. A calcium-dependent transient outward current inXenopus, laevis oocytes.Proc. R. Soc. London B 215:491–497
Miledi, R., Parker, I. 1984. Chloride current induced by, injection of calcium intoXenopus oocytes.J. Physiol. (London) 357:173–183
Mimura, T., Tazawa, M. 1983. Effects of intracellular Ca2+ on membrane potential and membrane resistance in tonoplast-free cells ofNitellopsis obtusa..Protoplasma 118:49–55
Mullins, L.J. 1962. Efflux of chloride ions during the action potential ofNitella.Nature (London) 196:986–987
Oda, K. 1976. Simultaneous recording of potassium and chloride effluxes during an action potential inChara corallina.Plant Cell Physiol. 17:1085–1088
Ohkawa, T., Kishimoto, U. 1977. Breakdown phenomena in theChara membrane.Plant Cell Physiol. 18:67–80
Okazaki, Y., Tazawa, M. 1986a. Involvement of calcium ion in turgor regulation upon hypotonic treatment inLamprothamnium succinctum.Plant Cell Environ. 9:185–190
Okazaki, Y., Tazawa, M. 1986b., Effect of calcium ion on cytoplasmic streaming during turgor regulation in a brackish water charophytaLamprothamnium succinctum.Plant Cell Environ. 9:491–494
Owen, D.G., Segal, M., Barker, J.L. 1984. A Ca-dependent Cl− conductance in cultured mouse spinal cord neurones.Nature (London) 311:567–570
Robinson, K.R. 1979. Electrical currents through full-grown and maturingXenopus oocytes.Proc. Natl. Acad. Sci. USA 76:837–841
Shiina, T., Tazawa, M. 1987. Demonstration and characterization of Ca2+ channel in tonoplast-free cells ofNitellopsis obtusa.J. Membrane Biol. 96:263–276
Shimmen, T., Tazawa, M. 1983. Activation of K+-channel in membrane excitation ofNitella axilliformis.Plant Cell Physiol. 24:1511–1524
Tazawa, M., Kikuyama, M., Shimmen, T. 1976. Electric characteristics and cytoplasmic streaming of Characeae cells lacking tonoplast.Cell Struct. Function 1:165–176
Tazawa, M., Kishimoto, U. 1968. Cessation of cytoplasmic streaming ofChara internodes during action potential.Plant Cell Physiol. 9:361–368
Tominaga, Y., Shimmen, T., Tazawa, M. 1983. Control of cytoplasmic streaming by extracellular Ca2+ in permeabilizedNitella cells.Protoplasma 116:75–77
Tsutsui, I., Ohkawa, T., Nagai, R., Kishimoto, U. 1986. Inhibition of Cl− channel activation inChara corallina membrane by lanthanum ion.Plant Cell Physiol. 27:1197–1200
Tsutsui, I., Ohkawa, T., Nagai, R., Kishimoto, U. 1987a. Role of calcium ion in the excitability and electrogenic pump activity of theChara corallina membrane: I. Effects of La3+, verapamil, EGTA, W-7, and TFP on the action potential.J. Membrane Biol. 96:65–74
Tsutsui, I., Ohkawa, T., Nagai, R., Kishimoto, U. 1987b. Role of calcium ion in the excitability and electrogenic pump activity of theChara corallina membrane: II. Effects of La3+, EGTA, and calmodulin antagonists on the current-voltage relation.J. Membrane Biol. 96:75–84
Tyerman, S.D., Findlay, G.P., Paterson, G.J. 1986a. Inward membrane current inChara inflata: I. A voltage- and time-dependent Cl− components.J. Membrane Biol. 89:139–152
Tyerman, S.D., Findlay, G.P., Paterson, G.J. 1986b. Inward membrane current inChara inflata: II. Effects of pH, Cl−, channel-blockers and NH +4 , and significance for the hyperpolarized state.J. Membrane Biol. 89:153–161
Williamson, R.E., Ashley, C.C. 1982. Free Ca2+ and cytoplasmic streaming in the algaChara.Nature (London) 296:647–650
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shiina, T., Tazawa, M. Ca2+-activated Cl− channel in plasmalemma ofNitellopsis obtusa . J. Membrain Biol. 99, 137–146 (1987). https://doi.org/10.1007/BF01871233
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01871233