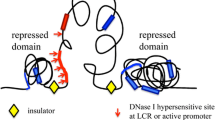
Summary
Calculations of DNA angular parameters in 50 eukaryotic sequences reveal regions of large conformational deviations from ideal DNA around regulatory sites. Frequently, discrete peaks of structural variation are present upstream of genes. Known regulatory regions often include variants of consensus sequences. Thus, imprecise sequences and structures are recognized within large genomic stretches. The existence of structurally “wrinkled” regions in the vicinity of regulatory sequences is likely to facilitate greatly their recognition by proteins and enzymes.
Similar content being viewed by others
References
Banerji J, Olson L, Schaffner W (1983) A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell 3:729–749
Barta A, Richards RI, Baxter JD, Shine J (1981) Primary structure and evolution of rat growth hormone gene. Proc Natl Acad Sci USA 78:4867–4871
Benoist C, Chambon P (1981) In vivo sequence requirements of the SV40 early promoter region. Nature 290:304–310
Benoist C, O'Hare K, Breathnach R, Chambon P (1980) The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res 8:127–142
Breathnach R, Chambon P (1981) Organization and expression of eukaryotic split genes coding for proteins. Annu Rev Biochem 50:349–383
Calladine CR (1982) Mechanics of sequence-dependent stacking of bases in B-DNA. J Mol Biol 161:343–352
Cochet M, Chang ACY, Cohen SN (1982) Characterization of the structural gene and putative 5′-regulatory sequences for human proopiomelanocortin. Nature 297:335–339
Davidson EH, Jacobs HT, Britten RJ (1983) Very short repeats and coordinate induction of genes. Nature 301:468–471
de Villier J, Schaffner W (1981) A small segment of polyoma virus DNA enhances the expression of a cloned β-globin gene over a distance of 1400 base pairs. Nucleic Acids Res 9:6251–6264
Dickerson RE (1983) Base sequence and helix structure variation in B- and A-DNA. J Mol Biol 166:419–441
Dickerson RE, Drew HR (1981a) Structure of a B-DNA dodecamer. II. Influence of base sequence on helix structure. J Mol Biol 149:761–786
Dickerson RE, Drew HR (1981b)Kinematic model for B-DNA. Proc Natl Acad Sci USA 78:7318–7322
Donahue TF, Daves RS, Lucchini G, Fink GR (1983) A short nucleotide sequence required for regulation of His4 by the general control system of yeast. Cell 32:89–98
Donehower LA, Huang AL, Hager GL (1981) Regulatory and coding potential of the mouse mammary tumor virus. J Virol 37:226–238
Drew HR, Dickerson RE (1981) Structure of B-DNA dodecamer III. Geometry of hydration. J Mol Biol 151:535–556
Drew HR, Takano T, Itakura K, Dickerson RE (1980) High salt d(CpGpCpG) a left-handed 2′ DNA double helix. Nature 286:567–573
Drew HR, Wing AM, Takano T, Broka C, Tanaka S, Itakura K, Dickerson RE (1981) Structure of B-DNA dodecamer: conformation and dynamics. Proc Natl Acad Sci USA 78:2179–2183
Gillies SD, Morrison SL, Oi VT, Tonegawa S (1983) A tissuespecific transcription enhancer element is located in the major intron of a rearranged immunoglobin heavy chain gene. Cell 3:717–728
Gorman CM, Merlino GT, Willingham MC, Pastan I, Howard BH (1982) The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA mediated transfection. Proc Natl Acad Sci USA 79:6777–6781
Groudine M, Eisenman R, Weintraub H (1981) Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature 292:311–317
Gruss P, Dhar R, Khoury G (1981) Simian virus 40 tandem repeated sequences as an element of the early promoter. Proc Natl Acad Sci USA 78:943–947
Guarente L (1984) Yeast promoters: positive and negative elements. Cell 36:799–800
Hearing P, Shenk T (1983) The adenovirus type 5 EIA transcriptional control region contains a duplicated enhancer element. Cell 33:695–703
Hen R, Borrelli E, Sassone-Corsi P, Chambon P (1983) An enhancer element is located 340 base pairs upstream from the adenovirus-2 E1A capsite. Nucleic Acids Res 11:8747–8760
Hinnebusch AG, Fink GR (1983) Repeated DNA sequences from His 1 also occur at several other co-regulated genes inSaccharomyces cerevisiae. J Biol Chem 258:5238–5247
Holmgren R, Corces V, Morimoto R, Blackman R, Meselson M (1981) Sequence homologies in the 5′ regions of fourDrosophila heat shock genes. Proc Natl Acad Sci USA 78:3775–3778
Ingolia TD, Craig EA (1981) Primary sequence of the 5′ flanking regions of theDrosophila heat shock genes in chromosome subdivision 67B. Nucleic Acids Res 9:1627–1642
Jolly DJ, Esty AC, Subramani S, Friedman T, Verma IM (1983) Elements in the long terminal repeat of murine retroviruses enhance stable transformation by thymidine kinase gene. Nucleic Acids Res 11:1855–1872
Jones KA, Tjian R (1984) Essential contact residues within SV40 large T antigen binding sites I and II identified by alkylation interference. Cell 36:155–162
Jongstra J, Reudelhuber TL, Oudet P, Benoist C, Chae C-B, Jeltsch J-M, Mathis DJ, Chambon P (1984) Induction of altered chromatin structures by simian virus 40 enhancer and promoter elements. Nature 307:708–714
Karch F, Torok I, Tissieres AJ (1981) Extensive regions of homology in front of the two hsp70 heat shock variant genes inDrosophila melanogaster. J Mol Biol 148:219–230
Kriegler M, Botchan M (1983) Enhanced transformation by a simian virus 40 recombinant virus containing a Harvey murine sarcoma virus long terminal repeat. Mol Cell Biol 3:325–339
Laimins LA, Khoury G, Gorman C, Howard B, Gruss P (1982) Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci USA 79:6453–6457
Lennon GG, Nussinov R (1984) Homonyms, synonyms and mutations of the sequence/structure vocabulary. J Mol Biol 175:425–430
Levinson B, Khoury G, Vande Woude G, Gruss P (1982) Activation of SV40 genome by 72-base pair tandem repeats of Moloney sarcoma virus. Nature 295:568–572
Lu P, Cheung S, Arndt K (1983) Possible molecular detent in the DNA structures at regulatory sequences. J Biomol Struct Dyn 1:509–521
Luciw PA, Bishop JM, Varmus HE, Capecchi MA (1983) Location and function of retroviral SV40 sequences that enhance biochemical transformation after microinjection of DNA. Cell 33:705–716
Lusky M, Berg L, Weiher H, Botchan M (1983) Bovine papilloma virus contains an activator of gene expression at the distal end of the early transcription unit. Mol Cell Biol 3:1108–1122
McKnight SL, Kingsbury R (1982) Transcriptional control of a eukaryotic protein-coding gene. Science 217:316–324
Nickol J, Behe M, Felsenfeld G (1982) Effect of the B-Z transition in poly (dG-m5 dC) on nucleosome formation. Proc Natl Acad Sci USA 79:1771–1775
Nordheim A, Rich A (1983) Negatively supercoiled simian virus 40 DNA contains DNA segments within transcriptional enhancer sequences. Nature 303:674–679
North G (1984) Latterday lessons of lambda and lac. Nature 308:687–688
Nussinov R, Shapiro B, Lipkin L, Maizel JV (1984) DNAase I hypersensitive sites may be correlated with genomic regions of large structural variations. J Mol Biol 177:591–607
Patel DJ, Kozlowski SA, Bhatt R (1983) Sequence dependence of base pair stacking in right-handed DNA in solution: proton nuclear Overhauser effect NMR measurements. Proc Natl Acad Sci USA 80:3908–3912
Payvar R, DeFranco D, Firestone GL, Edgar B, Wrange O, Okret S, Gustafsson J-A, Yamamoto K (1983) Sequence-specific binding of glucocorticoid receptor to MTV-DNA at sites within and upstream of the transcribed region. Cell 35:381–392
Palham HRB (1982) A regulatory upstream promoter element in theDrosophila hsp 70 heat shock gene. Cell 30:517–528
Pribnow D (1975) Nucleotide sequence of an RNA polymerase binding site at an early T7 promoter. Proc Natl Acad Sci USA 72:784–788
Queen C, Baltimore D (1983) Immunoglobin gene transcription is activated by downstream sequence elements. Cell 3:741–748
Rosenthal N, Kress M, Gruss P, Khoury G (1983) The Bk viral enhancer and a human cellular homologue. Science 222:749–755
Schaller H, Gray C, Herrmann K (1975) Nucleotide sequence of an RNA polymerase binding site from the DNA of bacteriophage Fd. Proc Natl Acad Sci USA 2:737–741
Shermoen AW, Beckendorf SK (1982) A complex of interacting DNAase I-hypersensitive sites near theDrosophila glue protein gene Sgs4. Cell 29:601–607
Struhl K (1982) Regulatory sites for His3 gene expression in yeast. Nature 300:284–286
Tyndall C, La Mantia G, Thacker CM, Favaloro J, Kamen R (1981) A region of the polyoma virus genome between the replication origin and late protein coding sequences is required in cis for both early gene expression and viral DNA duplication. Nucleic Acids Res 9:6231–6250
Weeks DL, Jones NC (1983) E1 A control of gene expression is mediated by sequence 5′ to the transcriptional starts of the early viral genes. Mol Cell Biol 3:1222–1234
Weiner H, Konig M, Gruss P (1983) Multiple point mutations affecting the simian virus 40 enhancer. Science 219:626–631
Wells RJ, Blakesley RW, Hardies SC, Horn GT, Larson JE, Selsing E, Burd JF, Chan HW, Dodgson JB, Jensen KF, Nes IF, Wartell RM (1977) CRC Crit Rev Biochem 4:305–340
Wu C (1980) The 5′ ends ofDrosophila heat shock genes in chromatin are sensitive to DNAase I. Nature 286:854–860
Zalkin H, Yanofsky C (1982) Yeast gene TRP5: structure, function, regulation. J Biol Chem 257:1491–1500
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nussinov, R. Structural wrinkles and the genomic regulatory sites of eukaryotes. J Mol Evol 22, 150–159 (1985). https://doi.org/10.1007/BF02101693
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02101693