Abstract.
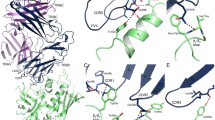
Pathogenic bacteria have evolved a wide variety of toxins to invade and attack host organisms. In particular, strains of the bacteria Staphylococcus aureus and Streptococcus pyogenes produce a family of pyrogenic toxin superantigens (PTSAgs) that can cause illness, e.g., toxic shock syndrome, or synergize with a number of other immune system disorders. The PTSAgs are all similar in size and have a conserved two-domain tertiary fold despite minimal amino acid sequence identity. The tertiary structure of PTSAg domain 1 is similar to the immunoglobulin binding motif of streptococcal proteins G and L. PTSAg domain 2 resembles members of the oligosaccharide/oligonucleotide binding fold family that includes the B subunits of the AB5 heat-labile enterotoxins, cholera toxin, pertussis toxin, and verotoxin. The strong structural homology between the pyrogenic toxins and other bacterial proteins suggests that the PTSAgs evolved through the recombination of two smaller β-strand motifs.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 7 March 2000 / Accepted: 18 August 2000
Rights and permissions
About this article
Cite this article
Mitchell, D., Levitt, D., Schlievert, P. et al. Structural Evidence for the Evolution of Pyrogenic Toxin Superantigens. J Mol Evol 51, 520–531 (2000). https://doi.org/10.1007/s002390010116
Published:
Issue Date:
DOI: https://doi.org/10.1007/s002390010116