Abstract
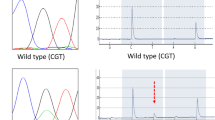
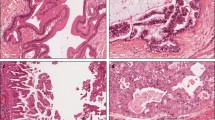
Mucin-producing tumors (MPTs) of the pancreas show a variety of clinical characteristics, including massive production of mucin in the pancreatic duct, dilatation of the main pancreatic duct, and a better prognosis than common invasive ductal carcinoma (IDC). These characteristics suggest that MPTs and IDCs have different cytomolecular backgrounds. The present study was designed to assess the differences in cytomolecular background between MPTs and IDCs, especially the differences in Ki-ras point mutation (PM) and wild and mutant typep53 expression. Cytomolecular backgrounds were compared in a 13 MPTs [8 carcinomas (MPCas) and 5 benign tumors (MPBTs)] and 36 IDCs. Cytomolecular studies included the evaluation of Ki-ras PM and the expression of Ki-ras p21, wild-typep53 (w-p53), and mutant-typep53 (m-p53). Ki-ras PM was assessed by the allele-specific oligonucleotide dot blot hybridization method, and the expression of p21 andp53 was assessed by an immunohistochemical staining method with monoclonal antibodies. Ki-ras PM was seen in 97% of IDCs and in 77% of MPTs (100% of MPCas and 40% of MPBTs), and MPBTs showed a significantly lower incidence of Ki-ras PM (versus IDC,P < 0.01). Guanine-Guanine-Thymine (GGT) to Guanine-Adenine-Thymine (GAT) mutation was seen in 55% of IDCs and 62% MPTs (87% of MPCas and 20% of MPBTs), and MPCas showed a significantly higher incidence of percent GAT mutation (versus IDC,P < 0.05). Ki-ras p21 was expressed in 43% of IDCs and in 31 % of MPTs (50% of MPCas and 20% of MPBTs). w-p53 and m-p53 were expressed in 51 % and 78% of IDCs and in 54% and 62% of MPTs (38% and 63% of MPCas and 20% and 60% of MPBTs), respectively. In MPBTs, hyperplasias showed higher rates of p21, w-p53, and m-p53 expression than cystadenomas. The study suggested that GAT mutation may be involved in the tumorigenesis of MPTs. It is suggested that MPBTs, especially hyperplasias, may be classified as low-grade malignancies like MPCas.
Similar content being viewed by others
References
Ohashi K, Takagi K (1988) An ERCP classification of pancreatic cancer. In: Maruyama M, Kimura K (eds) Review of clinical research in gastroenterology. Igaku-Shoin, Tokyo, pp 221–232
Morohoshi T, Kanda M, Asanuma K, Kloppel G (1989) Intraductal papillary neoplasms of the pancreas: A clinicopathologic study of six patients. Cancer 64:1329–1335
Conley CR, Scheithauer BW, Weiland LH, van Heerden JA (1987) Diffuse intraductal papillary adenocarcinoma of the pancreas. Ann Surg 205:246–249
Smith RC, Kneale K, Goulston K (1986) In situ carcinoma of the pancreas. Aust NZ J Surg 56:369–373
Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M (1988) Most human carcinomas of the exocrine pancreas contain mutant c-Ki-ras genes. Cell 53:549–554
Smit VT, Boot AJ, Smits AM, Fleuren GT, Cornelisse CJ, Bos JL (1988) K-ras codon 12 mutations occur very frequently in pancreatic adenocarcinomas. Nucleic Acids Res 16:7773–7782
Grunewald K. Lyons J, Frohlich A, Feichtinger H, Weger RA, Schwab G, Janssen JWG, Bartram CR (1989) High frequency of Ki-ras codon 12 mutations in pancreatic adenocarcinomas. Int J Cancer 43:1037–1041
Nagata Y, Abe M, Motojima K, Nakayama E, Shiku H (1990) Frequent glycine to asparatic acid mutations at codon 12 of c-Ki-ras gene in human pancreatic carcinomas in Japanese. Jpn J Cancer Res 81:135–140
Barton CM, Staddon SL, Hughes CM, Hall PA, O'Sullivan C, Kloppel G, Theis B, Russel RCG, Neoptolemos J, Williamson RCN, Lane DP, Lemonine NR (1991) Abnormalities of the p53 tumor suppressor gene in human pancreatic cancer. Br J Cancer 64:1076–1082
Hinds P, Finlay C, Levine AJ (1989) Mutation is required to activate the p53 gene for cooperation with the ras oncogene and transformation. J Virol 63:739–746
Vojtesek B, Bartek J, Midgley CA, Lane DP (1992) An immunochemical analysis of the human nuclear phosphoprotein p53. New monoclonal antibodies and epitope mapping using recombinant p53. J Immunol Methods 151:237–244
Gannon JV, Greaves R, Iggo R, Lane DP (1990) Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J 9:1595–1602
Tada M, Omata M, Ohto M (1991) Ras gene mutations in intraductal papillary neoplasms of the pancreas. Cancer 67:634–637
Yanagisawa A, Kato Y, Ohtake K, Kitagawa T, Ohashi K, Hori M, Takagi K, Sugano H (1991) c-Ki-ras point mutations in ductectasic-type mucinous cystic neoplasms of the pancreas. Jpn J Cancer Res 82:1057–1060
Lemoine NR, Jain S, Hughes CM, Staddon SL, Maillet B, Hall PA, Klöppel G (1992) Ki-ras oncogene activation in preinvasive pancreatic cancer. Gastroenterol 102:230–236
Hoshi T, Imai M, Ogawa K (1994) Frequent K-ras mutations and absence of p53 mutations in mucin-producing tumors of the pancreas. J Surg Oncol 55:84–91
Motojima K, Urano T, Nagata Y, Shiku H, Tsunoda T, Kanematsu T (1991) Mutations in the Kirsten-ras oncogene are common but lack correlation with prognosis and tumor stage in human pancretic carcinoma. Am J Gastroenterol 86:1784–1788
Scarpa A, Capelli P, Villanueva A, Zamboni G, Lluisi F, Accolla R, Matiuzzi G, Capella G (1994) pancreatic cancer in Europe: Ki-ras gene mutation pattern shows geographical differences. Int J Cancer 57:167–171
Mariyama M, Kishi K, Nakamura K, Obata H, Nishimura S (1989) Frequency and types of point mutation at the 12th codon of the c-Ki-ras gene found in pancreatic cancers from Japanese patients. Jpn J Cancer Res 80:622–626
Richaert F, Cremer M, Deviere J, Travares L, Lambilliotte JP, Schroder S, Wurbs D, Kloppel G (1991) Intraductal mucinhypersecreting neoplasms of the pancreas. A clinicopathologic study of eight patients. Gastroenterol 101:512–519
Eliyahu D, Michalovitz D, Eliyahu S, Pinhasi-Kimhi O, Oren M (1989) Wild-type p53 can inhibit oncogene-mediated focus formation. Proc Natl Acad Sci USA 86:8763–8767
Porter PL, Grown AM, Kramp SG, Coltera MD (1992) Widespread p53 overexpression in human malignant tumors: An immunohistochemical study using methacarn-fixed, embedded tissue. Am J Pathol 140:145–153
Van den Berg FM, Tiggers AJ, Schipper MEI, Den Hartog-Jager FCA, Krofs WGM, Walboomers JMM (1989) Expression of the nuclear oncogene p53 in colonic tumours. J Pathol 157:193–199
Mercer WE, Nelson D, Deleo AB, Old LJ, Baserga R (1982) Microinjection of monoclonal antibody to protein p53 inhibits serum-induced DNA synthesis in 3T3 cells. Proc Natl Acad Sci USA 79:6309–6312
Herskowitz I (1987) Functional inactivation of genes by dominant negative mutations. Nature 329:219–222
Iwaya K, Tsuda H, Hiraide H, Tamaki K, Tamakuma S, Fukutomi T, Mukai K. Hirohashi S (1991) Nuclear p53 immunoreaction associated with poor prognosis of breast cancer. Jpn J Cancer Res 82:835–840
Martin HM, Filipe MI, Morris RN, Lane DP, Silvester I (1992) p53 expression and prognosis in gastric carcinoma. Int J Cancer 50:859–862
Motojima K, Furui J, Kohara N, Ito T, Kanematsu T (1994) Expression of p53 protein in gastric carcinomas is not independently prognostic. Surgery 116:890–895
Lee CS, Rush M, Charalambous D, Rode J (1993) Immunohistochemical demonstration of the p53 tumour suppressor gene product in cancer of the pancreas and chronic pancreatitis. J Gastroenterol Hepatol 8:465–469
Zhang SY, Ruggeri B, Agarwal P, Sorling AF, Obara T, Ura H, Namiki M, Klein-Szanto JP (1994) Immunohistochemical analysis of p53 expression in human pancreatic carcinomas. Arch Pathol Lab Med 118:150–154
Gallo O, Franchi A, Bianchi S, Boddi V, Giannelli E, Alajmo E (1995) p53 oncoprotein expression in parotid gland carcinoma is associated with clinical outcome. Cancer 75:2037–2044
Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AMM, Bos JL (1988) Genetic alterations during colorectal-tumor development. N Engl J Med 319:525–532
Baker SG, Preisinger AC, Jessup JM, Paraskeva C, Markowitz S, Willson JKV, Hamilton S, Vogelstein B (1990) p53 gene mutations occur in combination with 17 p allelic delections as late events in colorectal tumorigenesis. Cancer Res 50:7717–7722
Kozuka S, Sassa R, Masamoto K, Nagasawa S, Saga S, Hasegawa K, Takeuchi M (1979) Relation of pancreatic duct hyperplasia to carcinoma. Cancer 43:1418–1428
Compagno J, Oertel JE (1978) Mucinous cystic neoplasms of the pancreas with overt and latent malignancy (cystadenocarcinoma and cystadenoma). A clinicopathological study of 41 cases. Am J Clin Pathol 69:573–580
Yanagisawa A, Ohtake K, Ohashi K, Hori M. Kitagawa T, Sugano H, Kato Y (1993) Frequent c-Ki-ras oncogene activation in mucous cell hyperplasias of pancreas suffering from chronic inflammation. Cancer Res 53:953–956
Pellegata NS, Sessa F, Renault B, Bonato M, Leone BE, Solcia E, Ranzani GN (1994) K-ras and p53 gene mutations in pancreatic cancer: Ductal and nonductal tumors progress through different genetic lesions. Cancer Res 54:1556–1560
Dupont WD, Parl FF, Hartmann WH, Brinton LA, Winfield AC, Worrel JA, Schuyler PA, Plummer WD (1993) Breast cancer risk associated with proliferative breast disease and atypical hyperplasia. Cancer 71:1258–1265
Jen J, Powell SM, Papadopoulos N, Smith KJ, Hamilton SR, Vogelstein B. Kinzler KW (1994) Molecular determinants of dysplasia in colorectal lesions. Cancer Res 54:5523–5526
Author information
Authors and Affiliations
About this article
Cite this article
Nio, Y., Sato, Y., Song, M.M. et al. Ki-ras point mutation in codon 12 and expression ofp53 in mucin-producing tumor of the pancreas. J Hep Bil Pancr Surg 4, 83–90 (1997). https://doi.org/10.1007/BF01211347
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01211347