Summary
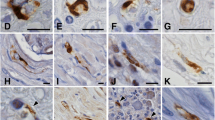
Aged-related spinal cord changes such as neuronal loss have been related to the degree of clinical severity of amyotrophic lateral sclerosis (ALS); morphological data on synapses are, however, wanting. Variations in synaptophysin (Sph) expression in aging and ALS were thus studied at the level of lower motor neurons in 40 controls with non-neurological diseases and 11 cases of ALS. Control sections of formalin fixed paraffin embedded cervical (C7/8), thoracic (T10) and lumbar spinal cord (L5) and C6, C7, C8 and L5 of ALS cases were stained with haematoxylin and eosin, luxol fast blue (LFB), and immunostained with a mouse monoclonal antibody against Sph. The neuropil of the anterior horn (AH) in all control cases demonstrated Sph positivity. A dot-like pattern of positivity of presynaptic terminals on soma of motor neurons and fine immunoreactivity along neuronal processes were observed. A significant reduction of Sph immunostaining was observed in the neuropil with increasing age and 3 different somatic patterns were seen: a-well preserved Sph reactivity around the soma and the proximal dendrites of histologically normal neurons; b-few chromatolytic neurons showing large numbers of dot-like presynaptic terminals around the cell body and in a “fused” pattern; c-intense, diffuse, and homogeneous reactivity of some neurons. Attenuation of Sph reactivity in the AH neuropil, to its complete loss, was observed in all ALS cases. In addition to patterns a-c, two additional microscopic findings were noted in ALS: d-chromatolytic neurons showing complete absence of Sph reactivity; e-absence of Sph reactivity around the soma and the proximal dendrites of histologically normal surviving neurons.
Our findings demonstrate that there is a decrease in Sph immunostaining with aging, thus suggesting an alteration in dendritic networks of the AH with aging. Changes in the pattern of Sph immunoreactivity in cell bodies may represent synaptic plasticity and/or degeneration. Reinnervation may also be a possible mechanism as a response to neuronal loss in oldest control cases. Sph reactivity results may thus lend support to the presence of superimposed aging components in ALS cases which may give an insight into explaining the increasing severity of the disease which is encountered with advancing age.
Similar content being viewed by others
References
Adams I (1987) Comparison of synaptic changes in the precentral cerebral cortex of aging humans. A quantitative ultrastructural study. Neurobiol Aging 8: 203–212
Barnes CA (1994) Normal aging: regionally specific changes in hippocampal synaptic transmission. Trends Neurosci 17: 13–18
Calne DB, McGeer E, Eisen A, Spencer P (1986) Alzheimer's disease, Parkinson's disease, and motorneuron disease: abiotropic interaction between ageing and environment. Lancet 2: 1067–1070
Coleman PD, Flood DG (1987) Neuron number and dendritic extent in normal aging and Alzheimer's disease. Neurobiol Aging 8: 521–545
Cruz-Sánchez, Rossi ML, Buller JR, Carboni P, Fineron PW, Coakham HB (1991) Oligodendrogliomas: a clinical, histological, immunocytochemical and lectin binding study. Histopathology 19: 361–367
Cruz-Sánchez FF, Moral A, Belleroche de J, Rossi ML (1993) Amyotrophic lateral sclerosis brain banking: a proposal to standardize protocols and neuropathological diagnostic criteria. J Neural Transm 39: 215S-222S
Eisen A, Schulzer M, MacNeil M, Pant B, Mak E (1993) Duration of amyotrophic lateral sclerosis is age dependent. Muscle Nerve 16: 27–32
Ferrer I, Roid C, Espino A et al (1991) Dementia of frontal lobe type and motor neuron disease. A Golgi study of frontal cortex. J Neurol Neurosurg Psychiatry 54: 932–934
Hillman DE, Chen S (1985) Plasticity in the size of presynaptic and postsynaptic membrane specializations. In: Cotman CW (ed) Synaptic plasticity. Guilford Press, New York, pp 39–76
Hirano A (1982) Aspect of the ultrastructure of amyotrophic lateral sclerosis. In: Rowland LP (ed) Human motor neuron diseases. Raven Press, New York, pp 75–88
Ikemoto A, Kawanami T, Llena JF, Hirano A (1994) Immunocytochemical studies on synaptophysin in the anterior horn of lower motor neuron diseases. J Neuropathol Exp Neurol 53: 196–201
Ince PG, Slade J, Chinnery RM, McKenzie J, Roystone C, Roberts GW, Shaw PJ (1995) Quantitative study of synaptophysin immunoreactivity of cerebral cortex and spinal cord in motor neuron disease. J Neuropathol Exp Neurol 54: 673–679
Iwatsubo T, Kuzuhara S, Kanemitsu A et al (1990) Corticofugal proyections to the motor nuclei of the brainstem and spinal cord in humans. Acta Neuropathol (Berl) 40: 309–312
Kato T, Hirano A, Donnenfeld H (1987) A Golgi study of the large anterior horn cells of the lumbar cords in normal spinal cords and in amyotrophic lateral sclerosis. Acta Neuropathol (Berl) 75: 34–40
Kato T, Hirano A, Kurland LT (1987) Asymmetric involvement of the spinal cord involving both large and small anterior horn cells in a case of familial amyotrophic lateral sclerosis. Clin Neuropathol 6: 67–70
Kawanami T, Ikemoto A, Llena JF, Hirano A (1993) Synaptophysin immunoreactivity in the anterior horn cells in amyotrophic lateral sclerosis (Abstract). Can J Neurol Sci 20: 75
Masliah E, Mallory M, De Teresa R, Hansen LA (1991) Cortical and subcortical patterns of synaptophysin-like immunoreactivity in Alzheimer's disease. Am J Pathol 138: 235–246
Moral A, Cardozo A, Rossi M et al (1994) Spinal motorneurons in aging. A morphometrical and immunocytochemical study (Absract). Brain Pathol 4: 577
Palacin A, Cardozo A, Cardesa A, Cruz-Sánchez FF (1993) Brain banking and non-nervous tissue. J Neural Transm 39: 87–96
Peters A, Palay SL, Webster HDeF (1991) The neuropil. In: Peters A, Palay SL, Webster HDeF (eds) The fine structure of the nervous system, 3rd ed. Oxford University Press, New York, pp 356–385
Ravid R, Winblad B (1993) Brain banking in Alzheimer's disease: factors to match for, pitfalls and potentials. In: Corain B, Iqbal K, Nicolini M, Winblad B, Wiesniewski H, Zatta P (eds) Alzheimer's disease: advances in clinical and basic research. Wiley, New York, pp 213–218
Ravid R, Swaab DF, Zwieten van EJ, Salehi A (1995) Controls are what makes a brain bank go round. In: Cruz-Sánchez FF, Ravid R, Cuzner ML (eds) Neuropathological diagnostic criteria for brain banking. IOS Press, Amsterdam, pp 4–13 (Biomed Health Res 10)
Rossi ML (1994) Motor neuron disease: classical pathology. In: Williams AC (ed) Motor neuron diseases (ALS), chapter 15. Chapman & Hall, London, pp 307–341
Rossi ML, Pugh BC, Lafuente JV et al (1992) Neocortical changes in MND: a Golgi/immunohistochemical study (Abstract). Neuropathol Appl Neurobiol 18: 309
Sasaki S, Maruyama S (1994) Decreased synaptophysin immunoreactivity of the anterior horns in motor neuron disease. Acta Neuropathol (Berl) 87: 125–128
Shaw PJ (1994) Excitotoxicity and motor neurone disease. A review of the evidence. J Neurol Sci 124: 6S-13S
Shaw PJ, Ince PG, Johnson M, Perry EK, Candy JM (1991) The quantitative autoradiographic distribution of (3H)MK-801 binding sites in the normal human spinal cord. Brain Res 539: 164–168
Scheibel ME, Lindsay RD, Tomiyasu U, Scheibel AB (1975) Progressive dendritic changes in aging human cortex. Exp Neurol 47: 392–403
Scheibel ME, Lindsay RD, Tomiyasu U, Scheibel AB (1976) Progressive dendritic changes in the aging human limbic system. Exp Neurol 53: 420–430
Scheibel ME, Tomiyasu U, Scheibel AB (1977) The aging human Betz cell. Exp Neurol 56: 598–609
Schoen JHR (1964) Comparative aspects of the descending fibre system in the spinal cord. In: Eccles JC, Schade JP (eds) The organization of the spinal cord. Elsevier, Amsterdam, pp 203–22 (Prog Brain Res 11)
Smith MC (1960) Nerve fibre degeneration in the brain in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 23: 269–282
Tomlinson BE, Irving D (1977) The number of limbic motor neurons in the lumbarsacral cord throughout life. J Neurol Sci 34: 213–219
Tsukagoshi H, Yanagisaba N, Oguchi K, Murakami T (1979) Morphometric quantification of the cervical limb motor cells in controls and in amyotrophic lateral sclerosis. J Neurol Sci 41: 287–97
Wiedenmann W, Franke W (1985) Identification and localization of synaptophysin, an integral membrane glyco protein of Mr 38,000 characteristic of presynaptic vesicles. Cell 41: 1017–1028
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cruz-Sánchez, F.F., Moral, A., Rossi, M.L. et al. Synaptophysin in spinal anterior horn in aging and ALS: an immunohistological study. J. Neural Transmission 103, 1317–1329 (1996). https://doi.org/10.1007/BF01271192
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01271192