Summary
The results of sorption measurements for gallium (III) and iodide ion traces onto tin(II) hydroxide from aqueous heterogeneous systems are given. Since only tagged sorbate was used (67Ga and 1311), in the determination of sorption by radioactive tracer method, it was necessary to establish the pH interval of tin(II) hydroxide in/stability. This was done by a turbidimetric method and the pH region of instability between 3 and 9 was found.
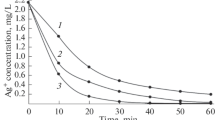
The sorption results indicate a difference in sorption ability of gallium(III) and iodide as it appears from the dependence of the amount sorbed on contact time between heterogeneous reactants (sorbentsorbate). The amounts sorbed increase with increasing contact times at higher pH values for Ga(III) but remain unaffected for iodide sorption. Thus the sorption of iodide ions seems to be restricted to the solid surface while the sorption results for gallium(III) indicate the diffusion of sorbate into sorbent as a possible parallel mechanism. Those processes could be taken as compatible with the porous-double-layer mechanism which has been proposed in literature for aqueous heterogeneous oxide systems.
Zusammenfassung
In der Arbeit werden Ergebnisse von Sorptionsmessungen für Ga3+- und j-Spuren an Zinn(II)-Hydroxid aus wäßrigen heterogenen Systemen berichtet. Zur analytischen Erfassung wurden radioaktives67Ga und 131 J verwendet. Die Stabilität von Zinn(II)-Hydroxid wurde durch Trübungsmessungen untersucht. Im pH-Bereich von 3–9 ist das Zinn(II)-Hydroxid als kolloide Suspension instabil.
Ga3+ und J− verhalten sich bei der Adsorption unterschiedlich. Die adsorbierte Menge steigt mit zunehmender Kontaktzeit bei hohen pH-Werten von Gallium, bleibt jedoch für Jod unverändert. Die Unterschiede werden gedeutet durch die Annahme, daß Jod nur an der festen Oberfläche adsorbiert wird, während die Galliumionen auch in das Sorbens diffundieren können. Man könnte die Ergebnisse als verträglich mit einem porösen Doppelschichtmechanismus ansehen; ein solcher Mechanismus ist in der Literatur für wäßrige heterogene Oxidsysteme vorgeschlagen worden.
Similar content being viewed by others
References
Hingston, F. J., A. M. Posner, andJ. P. Quirk, Disc. Faraday Soc.52, 334 (1971).
Sykora, S. andZ. Kolarik, Coll. Czech. Chem Commun.29, 1350 (1964)
Mun, A. I., A. B. Bekturov, andA. L. Mazurova, Tr. Inst. Khim. Nauk, Akad. Nauk Kaz. SSR25, 59 (1969).
Goodall, C. A. andR. Z. Moore, J. Inorg. Nucl. Chem.11, 290 (1959).
Maass, R., J. Alvarez, andC. Arriaga, Int. J. Appl. Radiat. Isotopes18, 653 (l967).
Monroe, L. A., W. L. Thompson, N. S. Anderton, andJ. A. Burdine, J. Nucl. Med.15, 192 (1974).
Verwey, E. J. W. andJ. Th. G. Overbeek with collaboration ofK. Van Nes, Theory of stability of lyophobic colloids (New York, Amsterdam, London and Brussels 1948).
Hamaker, H. C., Physica (Amsterdam)4, 1058 (1937).
Hiemenz, P. C., J. Chem. Educ.49, 164 (1972).
Težak, B., E. Matijevic, andK. Schulz, J. Phys. Colloid Chem.55, 1557 (1951).
Coryell, C. D. andN. Sugarman, Radiochemical Studies. The fission products. Book 1, Parts I, 11, III, IV, Papers 12, 13 and 14 (New York).
Ŝipalo-Žul ević, J. andR. H. H. Wolf, Mikrochimica Acta (Wien)315 (1973).
Plotnikov, V. I., Zhurnal Neorg. Khimii8, 1761 (1958).
Kolarik, Z. andV. Kourim, Coll. Czech. Chem. Commun.25, 1000 (1960).
Tadros, Th. F. andJ. Lyklema, J. Electroanal. Chem. Interfatial Electrochem.22, 9 (1969).
Lyklema, J., J. Electroanal. Chem. Interfatial Electrochem.18, 341 (1968).
Author information
Authors and Affiliations
Additional information
With 4 figures
Rights and permissions
About this article
Cite this article
Musić, S., Ŝipalo-Žuljević, J. & Vlatković, M. Sorption of trace concentrations of gallium(III) and iodide ions on tin(II) hydroxide. Colloid & Polymer Sci 255, 35–39 (1977). https://doi.org/10.1007/BF01449634
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01449634