Abstract
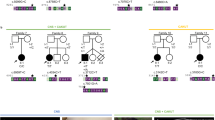
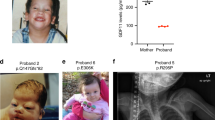
Fraser syndrome (OMIM 219000) is a multisystem malformation usually comprising cryptophthalmos, syndactyly and renal defects1. Here we report autozygosity mapping and show that the locus FS1 at chromosome 4q21 is associated with Fraser syndrome, although the condition is genetically heterogeneous. Mutation analysis identified five frameshift mutations in FRAS1, which encodes one member of a family of novel proteins related to an extracellular matrix (ECM) blastocoelar protein found in sea urchin. The FRAS1 protein contains a series of N-terminal cysteine-rich repeat motifs previously implicated in BMP metabolism, suggesting that it has a role in both structure and signal propagation in the ECM. It has been speculated that Fraser syndrome is a human equivalent of the blebbed phenotype in the mouse2, which has been associated with mutations in at least five loci including bl3. As mapping data were consistent with homology of FRAS1 and bl, we screened DNA from bl/bl mice and identified a premature termination of mouse Fras1. Thus, the bl mouse is a model for Fraser syndrome in humans, a disorder caused by disrupted epithelial integrity in utero.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Slavotinek, A.M. & Tifft, C.J. Fraser syndrome and cryptophthalmos: review of the diagnostic criteria and evidence for phenotypic modules in complex malformation syndromes. J. Med. Genet. 39, 623–633 (2002).
Winter, R.M. Fraser syndrome and mouse 'bleb' mutants. Clin. Genet. 37, 494–495 (1990).
Darling, S. & Gossler, A. A mouse model for Fraser syndrome? Clin. Dysmorphol. 3, 91–95 (1994).
Thomas, I.T. et al. Isolated and syndromic cryptophthalmos. Am. J. Med. Genet. 25, 85–98 (1986).
Warkany, J. & Schraffenberger, E. Congenital malformation of the eyes induced in rats by maternal vitamin A deficiency. Proc. Soc. Exp. Biol. 57, 49–52 (1944).
Dupe, V. et al. Essential roles of retinoic acid signaling in interdigital apoptosis and control of BMP-7 expression in mouse autopods. Dev. Biol. 208, 30–43 (1999).
Kruglyak, L., Daly, M.J., Reeve-Daly, M.P. & Lander, E.S. Parametric and nonparametric linkage analysis: a unified multipoint approach. Am. J. Hum. Genet. 58, 1347–1363 (1996).
Center, E.M. & Emery, K.E. Acidic glycosaminoglycans and laminin-1 in renal corpuscles of mutant blebs (my) and control mice. Histol. Histopathol. 12, 901–907 (1997).
Carter, T.C. Embryology of the Little and Bagg X-rayed mouse stock. J. Genet. 56, 401–435 (1959).
Gruneberg, H. The Genetics of the Mouse (Martinus Nijhoff, The Hague, 1952).
Arin, M.J. & Roop, D.R. Disease model: heritable skin blistering. Trends Mol. Med. 7, 422–424 (2001).
Winyard, P.J. et al. Deregulation of cell survival in cystic and dysplastic renal development. Kidney Int. 49, 135–146 (1996).
Muller, U. et al. Integrin α8β1 is critically important for epithelial–mesenchymal interactions during kidney morphogenesis. Cell 88, 603–613 (1997).
Sorenson, C.M., Rogers, S.A., Korsmeyer, S.J. & Hammerman, M.R. Fulminant metanephric apoptosis and abnormal kidney development in bcl-2-deficient mice. Am. J. Physiol. 268, F73–F81 (1995).
Dudley, A.T., Godin, R.E. & Robertson, E.J. Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme. Genes Dev. 13, 1601–1613 (1999).
Hodor, P.G., Illies, M.R., Broadley, S. & Ettensohn, C.A. Cell–substrate interactions during sea urchin gastrulation: migrating primary mesenchyme cells interact with and align extracellular matrix fibers that contain ECM3, a molecule with NG2-like and multiple calcium-binding domains. Dev. Biol. 222, 181–194 (2000).
Goretzki, L., Burg, M.A., Grako, K.A. & Stallcup, W.B. High-affinity binding of basic fibroblast growth factor and platelet-derived growth factor-AA to the core protein of the NG2 proteoglycan. J. Biol. Chem. 274, 16831–16837 (1999).
Matsui, M., Mizuseki, K., Nakatani, J., Nakanishi, S. & Sasai, Y. Xenopus kielin: a dorsalizing factor containing multiple chordin-type repeats secreted from the embryonic midline. Proc. Natl. Acad. Sci. USA 97, 5291–5296 (2000).
Constam, D.B. & Robertson, E.J. Regulation of bone morphogenetic protein activity by pro domains and proprotein convertases. J. Cell Biol. 144, 139–149 (1999).
Conley, C.A. et al. Crossveinless 2 contains cysteine-rich domains and is required for high levels of BMP-like activity during the formation of the cross veins in Drosophila. Development 127, 3947–3959 (2000).
Mellerio, J.E. Molecular pathology of the cutaneous basement membrane zone. Clin. Exp. Dermatol. 24, 25–32 (1999).
Nodder, S. & Martin, P. Wound healing in embryos: a review. Anat. Embryol. (Berl) 195, 215–228 (1997).
Arteaga-Solis, E. et al. Regulation of limb patterning by extracellular microfibrils. J. Cell Biol. 154, 275–281 (2001).
Davies, J.A. & Fisher, C.E. Genes and proteins in renal development. Exp. Nephrol. 10, 102–113 (2002).
Kunkel, L.M. et al. Analysis of human Y-chromosome-specific reiterated DNA in chromosome variants. Proc. Natl. Acad. Sci. USA 74, 1245–1249 (1977).
Roberts, C., Platt, N., Streit, A., Schachner, M. & Stern, C.D. The L5 epitope: an early marker for neural induction in the chick embryo and its involvement in inductive interactions. Development 112, 959–970 (1991).
Tidman, M.J. & Eady, R.A. Ultrastructural morphometry of normal human dermal-epidermal junction. The influence of age, sex, and body region on laminar and nonlaminar components. J. Invest. Dermatol. 83, 448–453 (1984).
Altschul, S.F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997).
Acknowledgements
We are grateful to N. Brown for help with histological sectioning. The work was supported by the Wellcome Trust (S.M.D., L.M.), the British Heart Foundation (P.J.S.), the Kidney Research Aid Fund and the National Kidney Research Fund (A.S.W.) and a Medical Research Council co-operative grant (R.M.W., P.J.S.) and was conducted within the London Genetics Knowledge Park.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
McGregor, L., Makela, V., Darling, S. et al. Fraser syndrome and mouse blebbed phenotype caused by mutations in FRAS1/Fras1 encoding a putative extracellular matrix protein. Nat Genet 34, 203–208 (2003). https://doi.org/10.1038/ng1142
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1142
This article is cited by
-
Fraser syndrome with limb reduction defect: a rare and unique anatomic variation
Surgical and Radiologic Anatomy (2024)
-
“Hypothesis: Patient with possible disturbance in programmed cell death”: further insights in pathogenicity and clinical features of Fraser syndrome
European Journal of Human Genetics (2023)
-
METTL3 promotes non-small cell lung cancer (NSCLC) cell proliferation and colony formation in a m6A-YTHDF1 dependent way
BMC Pulmonary Medicine (2022)
-
Deciphering the mutation spectrum in south Indian children with congenital anomalies of the kidney and urinary tract
BMC Nephrology (2022)
-
The term CAKUT has outlived its usefulness: the case for the prosecution
Pediatric Nephrology (2022)