Abstract
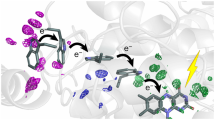
Protein photosensors from all kingdoms of life1,2 use bound organic molecules, known as chromophores, to detect light. A specific double bond within each chromophore is isomerized by light, triggering slower changes in the protein as a whole. The initial movements of the chromophore, which can occur in femtoseconds, are tightly constrained by the surrounding protein, making it difficult to see how isomerization can occur, be recognized, and be appropriately converted into a protein-wide structural change and biological signal. Here we report how this dilemma is resolved in the photoactive yellow protein (PYP). We trapped a key early intermediate in the light cycle of PYP at temperatures below −100 °C, and determined its structure at better than 1 Å resolution. The 4-hydroxycinnamoyl chromophore3,4 isomerizes by flipping its thioester linkage with the protein, thus avoiding collisions resulting from large-scale movement of its aromatic ring during the initial light reaction. A protein-to-chromophore hydrogen bond that is present in both the preceding dark state5 and the subsequent signalling state6 of the photosensor breaks, forcing one of the hydrogen-bonding partners into a hydrophobic pocket. The isomerized bond is distorted into a conformation resembling that in the transition state. The resultant stored energy is used to drive the PYP light cycle. These results suggest a model for phototransduction, with implications for bacteriorhodopsin7,8, photoactive proteins1,2, PAS domains9, and signalling proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Unger, V. M., Hargrave, P. A., Baldwin, J. M. & Schertler, G. F. X. Arrangement of rhodopsin transmembrane α-helices. Nature 389, 203–206 (1997).
Quail, P. H. et al. Phytochromes: photosensory perception and signal transduction. Science 268, 675–680 (1995).
Baca, M. et al. Complete chemical structure of photoactive yellow protein: novel thioester-linked 4-hydroxycinnamyl chromophore and photocycle chemistry. Biochemistry 33, 14369–14377 (1994).
Hoff, W. D. et al. Thiol ester-linked p-coumaric acid as a new photoactive prosthetic group in a protein with rhodopsin-like photochemistry. Biochemistry 33, 13959–13962 (1994).
Borgstahl, G. E. O., Williams, D. R. & Getzoff, E. D. 1.4 Å structure of photoactive yellow protein, a cytosolic photoreceptor: unusual fold, active site, and chromophore. Biochemistry 34, 6278–6287 (1995).
Genick,, K. et al. Structure of a protein photocycle intermediate by millisecond time-resolved crystallography. Science 275, 1471–1475 (1997).
Pebay-Peyroula, E., Rummel, G., Rosenbusch, J. P. & Landau, E. M. X-ray structure of bacteriorhodopsin at 2.5 Ångstroms from microcrystals grown in lipidic cubic phases. Science 277, 1676–1681 (1997).
Kimura, Y. et al. Surface of bacteriorhodopsin revealed by high-resolution electron crystallography. Nature 389, 206–211 (1997).
Kay, S. A. PAS, present, and future: clues to the origins of circadian clocks. Science 276, 753–754 (1997).
Meyer, T. E. Isolation and characterization of soluble cytochromes, ferredoxins and other chromophoric proteins from the halophilic phototrophic bacterium Ectothiorhodospira halophila. Biochim. Biophys. Acta 806, 175–183 (1985).
Sprenger, W. W., Hoff, W. D., Armitage, J. P. & Hellingwerf, K. J. The eubacterium Ectothiorhodospira halophila is negatively phototactic, with a wavelength dependence that fits the absorption spectrum of the photoactive yellow protein. J. Bacteriol. 175, 3096–3104 (1993).
Kim, M., Mathies, R. A., Hoff, W. D. & Hellingwerf, K. J. Resonance Raman evidence that the thioester-linked 4-hydroxycinnamyl chromophore of photoactive yellow protein is deprotonated. Biochemistry 34, 12669–12672 (1995).
Meyer, T. E., Tollin, G., Hazzard, J. H. & Cusanovich, M. A. Photoactive yellow protein from the purple phototrophic bacterium, Ectothiorhodospira halophila. Quantum yield of photobleaching and effects of temperature, alcohols, glycerol, and sucrose on kinetics of photobleaching and recovery. Biophys. J. 56, 559–564 (1989).
Hoff, W. D. et al. Measurement and global analysis of the absorbance changes in the photocycle of the photoactive yellow protein from Ectothiorhodospira halophila. Biophys. J. 67, 1691–1705 (1994).
Imamoto, Y., Kataoka, M. & Tokunaga, F. Photoreaction cycle of photoactive yellow protein from Ectothiorhodospira halophila studied by low-temperature spectroscopy. Biochemistry 35, 14047–14053 (1996).
Genick, U. K. et al. Active site mutants implicate key residues for control of color and light cycle kinetics of photoactive yellow protein. Biochemistry 36, 8–14 (1997).
Sheldrick, G. M. in Crystallographic Computing 7 (eds Watenpaugh, K. & Bourne, P.) (Oxford Press, Oxford, in the press).
Xie, A., Hoff, W. D., Kroon, A. R. & Hellingwerf, K. J. Glu 46 donates a proton to the 4-hydroxycinnamate anion chromophore during the photocycle of photoactive yellow protein. Biochemistry 35, 14671–14678 (1996).
van Brederode, M. E., Gensch, T., Hoff, W. D., Hellingwerf, K. J. & Braslavsky, S. E. Photoinduced volume change and energy storage associated with the early transformations of the photoactive yellow protein from Ectothiorhodospira halophila. Biophys. J. 68, 1101–1109 (1995).
Stowell, M. H. B. et al. Light-induced structural changes in photosynthetic reaction center: implications for mechanism of electron-proton transfer. Science 276, 812–816 (1997).
Williams, P. A. et al. Haem-ligand switching during catalysis in crystals of a nitrogen-cycle enzyme. Nature 389, 406–412 (1997).
Schlichting, I., Berendzen, J., Phillips, G. N. J & Sweet, R. M. Crystal structure of photolysed carbonmonoxy-myoglobin. Nature 371, 808–812 (1994).
Hajdu, J. & Anderson, I. Fast crystallography and time-resolved structures. Annu. Rev. Biophys. Biomol. Struct. 22, 467–498 (1993).
Bourgeois, D. et al. Feasibility and realization of single-pulse Laue diffraction on macromolecular crystals at ESRF. J. Synchrotron Rad. 3, 65–74 (1996).
Schertler, G. F. X., Lozier, R., Michel, H. & Oesterhelt, D. Chromophore motion during the bacteriorhodopsin photocycle: polarized absorption spectroscopy of bacteriorhodopsin and its M-state in bacteriorhodopsin crystals. EMBO J. 10, 2353–2361 (1991).
Hadfield, A. & Hajdu, J. Afast and portable microspectrophotometer for protein crystallography. J. Appl. Cryst. 26, 839–842 (1993).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Acknowledgements
We thank C. D. Mol and C. D. Putnam for testing the PYP crystals for sub-Ångstrom diffraction; B. E. Honig for discussing low-temperature approaches to the study of photosensors; J.Berendzen for discussing appropriate illumination conditions; C. L. Brooks III, B. N. Dominy, J.-L. Pellequer and D. A. Case for exploring possibilities of energy calculation; and T. Woo for growing the crystal used for microspectrophotometry. This research was supported by a grant from the N.I.H. (to E.D.G.) and a scholarship from Boehringer Ingelheim Fonds (for U.K.G.). This work is based on research conducted at the Stanford Synchrotron Radiation Laboratory (SSRL), which is funded by the Department of Energy, Office of Basic Energy Sciences. The Biotechnology Program is supported by the NIH, National Center for Research Resources, Biomedical Technology Program and the Department of Energy, Office of Biological and Environmental Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Genick, U., Soltis, S., Kuhn, P. et al. Structure at 0.85 Å resolution of an early protein photocycle intermediate. Nature 392, 206–209 (1998). https://doi.org/10.1038/32462
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/32462
This article is cited by
-
Identification of absolute geometries of cis and trans molecular isomers by Coulomb Explosion Imaging
Scientific Reports (2016)
-
Volume-conserving trans–cis isomerization pathways in photoactive yellow protein visualized by picosecond X-ray crystallography
Nature Chemistry (2013)
-
Estimation of the available free energy in a LOV2-Jα photoswitch
Nature Chemical Biology (2008)
-
Bacteriophytochromes in anoxygenic photosynthetic bacteria
Photosynthesis Research (2008)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.